First、 Physical Law
Physical methods can usually prepare copper powder with high purity and good sphericity.
1.Atomization method
Aerosolization method: using high-pressure inert gas, the resulting powder has low oxygen content and good sphericity, but the cost is relatively high. Commonly used in high-performance powder metallurgy and 3D printing of metal powders.
Water atomization method: using high-pressure water flow, fast cooling speed, irregular powder particles obtained (mostly in the form of flakes or tears), large specific surface area, and relatively high oxygen content. Low cost, commonly used for manufacturing diamond tools, friction materials, etc.
Principle: Molten copper liquid is sprayed out through a nozzle, crushed into small droplets using high-pressure gas (air, nitrogen, argon) or high-pressure water, and then condensed into spherical or nearly spherical powder under the action of surface tension.
Characteristics: High production efficiency, easy industrial large-scale production, and is one of the main methods for producing metal powders.
2.Vacuum evaporation condensation method
Principle: In a high vacuum environment, metal copper is heated and evaporated, and gaseous copper atoms are condensed into ultrafine powder on a low-temperature condensation wall.
Characteristics: It can prepare nanoscale, high-purity ultrafine copper powder, but the equipment is complex, the yield is low, and the cost is high. It is mainly used in laboratory research and high-precision fields.
Second, Chemical method
Chemical methods can prepare copper powder with finer particle size and diverse shapes, especially nano copper powder.
1.Liquid phase reduction method (the most commonly used chemical method)
Principle: Using soluble copper salts (such as copper sulfate and copper chloride) as raw materials and hydrazine hydrate, formaldehyde, sodium borohydride, ascorbic acid (vitamin C) or glucose as reducing agents, Cu ² ⁺ is reduced to Cu ⁰ atoms in the liquid phase and aggregated into powder.
Reaction example (using copper sulfate and hydrazine hydrate as examples):
2CuSO₄ + N₂H₄·H₂O + 4NaOH → 2Cu↓ + N₂↑ + 2Na₂SO₄ + 5H₂O
Characteristics: The operation is relatively simple, and the particle size and morphology of copper powder can be controlled by adjusting parameters such as reactant concentration, temperature, pH value, and additives (dispersants, protectants). It is the most commonly used method for laboratory and industrial production of ultrafine copper powder.
2.electrolysis method
Principle: Use copper plate as the anode and titanium or stainless steel plate as the cathode, and apply electricity in an electrolyte containing copper ions. The copper at the anode loses electrons and dissolves into Cu ² ⁺. Cu ² ⁺ obtains electrons at the cathode and is reduced to metallic copper, which is deposited in powder form on the cathode.
Characteristics: The obtained copper powder has high purity (up to 99.95% or more), dendritic structure, large specific surface area, and good compressibility. But the energy consumption is high and the cost is also high. It is an important method for producing copper powder for electrical materials.
3. Thermal decomposition method
Principle: Copper compounds (such as copper formate, basic copper carbonate, and carbonyl compounds of copper) undergo thermal decomposition at a specific temperature, producing copper powder and gas products. Characteristics: The powder has high purity, but the raw material cost is high and may produce harmful gases, making it less commonly used.
3、 Mechanical Law
ball milling
Principle: Metal copper blocks or copper shavings are placed together with grinding balls in a ball milling jar. Through the high-speed rotation or vibration of the ball mill, the grinding balls strongly impact, crush, and friction the copper material, causing it to repeatedly break and cold weld, ultimately refining it into powder
Features: Simple equipment, high output, low cost. But the powder shape is irregular, the particle size distribution is wide, it is easy to introduce impurities, and the energy consumption is high. Mainly used for producing flake copper powder (such as copper gold powder for coatings) or alloying copper with other elements.
The above are several mainstream methods for synthesizing copper powder, and there are other methods that can also be used to produce copper powder.
SAT NANO is a best supplier of copper powder in China, we can offer 50nm, 100nm, 500nm, 1-3um, 5um and so on, if you have any enquiry, please feel free to contact us at admin@satnano.com
Behind the flourishing development of many industries such as electronics and new energy, there is a seemingly insignificant but crucial material - conductive silver paste. Keyboards, mobile phones, tablets, solar panels, smart cards, RFID and other devices rely on them to achieve connections and fully function.
Silver has the highest conductivity among metals, with excellent conductivity, thermal conductivity, good chemical stability, and weldability, and is widely used in various fields of modern electronics industry. Whether it is an OLED flexible screen or an LCD screen, there are countless thin conductive lines inside the screen, which are printed with "conductive silver paste".

1. Composition of conductive silver paste
Conductive silver paste is usually a viscous paste prepared by mechanical mixing of micrometer sized metal silver particles, polymer binders, solvents, additives, etc.
Among them, silver powder is a conductive functional phase, and its intrinsic characteristics such as microstructure, morphology, size and distribution, surface activity, etc. have a significant impact on the conductivity of the final film layer.
Adhesive phase is the film-forming substance of conductive paste, which can be divided into two categories: inorganic adhesive phase and organic adhesive phase. Its main functions are twofold: firstly, it acts as a skeleton, bonding silver powder together to achieve the conductive function of wiring; The second is to act between the film layer and the substrate to achieve the bonding between the conductive film layer and the substrate.
Solvent is also an essential component of conductive paste, playing a role in dissolving resin and adjusting the viscosity of the paste.
In addition, additives also play an important role in conductive pastes, mainly including thixotropes, dispersants, leveling agents, antioxidants, etc., which improve the coating performance of the paste and the physicochemical properties of the film layer.
2. Conductive silver paste classified by curing method
Conductive silver paste can be divided into three categories based on the curing method: sintered conductive silver paste, low-temperature cured conductive silver paste, and UV cured silver paste.
A. Sintered conductive silver paste
Sintered conductive paste is made by mixing silver powder as the conductive phase, glass powder as the bonding phase, organic solvents and other additives as the organic carrier. This type of conductive silver paste is sintered into a film at a sintering temperature generally>500 ℃.
Sintered conductive silver paste is mainly used for high-temperature insulation substrates such as ceramics and glass. This type of conductive silver paste is commonly used in electronic components such as potentiometers and thick film circuits that can withstand high temperatures, such as MLCC internal electrodes, photovoltaic cell electrode printing, and PCB local conductive paths.
B. Low temperature curing conductive silver paste
Low temperature cured conductive silver paste is composed of silver powder, polymer organic system, organic solvent, and additives. The curing temperature is generally less than 300 ℃, and good adhesion of circuit wires to the substrate can be obtained using screen printing technology.
The main characteristics of low-temperature curing conductive silver paste are low curing temperature, high bonding strength, and stable electrical performance. It is suitable for conductive and thermally conductive bonding in low-temperature curing welding applications, such as quartz crystals, infrared pyroelectric detectors, piezoelectric ceramics, potentiometers, flash lamps, as well as shielding, circuit repairs, etc; It can also be used for conductive bonding in the wireless instrument industry.
Low temperature cured conductive silver paste can also be used for printing on various flexible substrates, such as PET, PC, PEN, PI, paper, thermoplastic elastomers, etc.
C.UV cured silver paste
The bonding in UV cured silver paste is more complex compared to the previous two, and should at least have UV curing monomers, photosensitizers, and crosslinking agents.
Its curing mechanism is also relatively complex. The photosensitizer is excited by ultraviolet light to become an activating molecule, which induces the copolymerization reaction of photosensitive polymers and ultimately crosslinks to form a network structure.
Due to the fact that film formation does not require heating, UV cured silver paste is often used as a thermal sensor for bonding and conducting components that do not require heating and curing.
Conductive silver paste, as one of the indispensable basic materials in the electronics industry, its performance optimization and technological innovation will directly affect the progress and development of the entire industry. With the continuous updating and progress of applications, we look forward to the wider application of conductive silver paste in the future.
SAT NANO is one of the best supplier of silver powder in China, we can offer nano particle and micron particle for client to do condutivity paste, if you have any enquiry, please feel free to contact us admin@satnano.com
The factors that affect the forming process mainly include the properties of the powder, the characteristics and effectiveness of additives, and the pressure, pressurization method, and pressurization speed during the pressing process. The properties of the powder mainly include the particle size, particle size distribution, particle shape, and moisture content of the powder.

First. The influence of powder properties on the pressing process
(1) The influence of the hardness and plasticity of metal powder itself. The hardness and plasticity of metal powder have a significant impact on the pressing process. Soft metal powder is easier to press than hard metal powder, which means that in order to obtain a certain density of compact, the pressing pressure required for soft metal powder is much smaller than that for hard metal powder. Soft metal powder deforms greatly during compression, increases the contact area between powders, and is easier to increase the compact density. Hard metal powders with poor plasticity must use forming agents during compression, otherwise they are prone to compression defects such as cracks.
(2) The influence of metal powder friction performance. Metal powder has a significant impact on the wear of molds, and the lifespan of molds is short when compressing hard metal powder.
(3) The influence of powder purity. The purity (chemical composition) of the powder has a certain impact on the pressing process. The higher the purity of the powder, the easier it is to press. When manufacturing high-density parts, the chemical composition of the powder has a significant impact on its formability, as impurities mostly exist in the form of oxides, and metal oxide powders are mostly hard and brittle and exist on the surface of the metal powder. During compression, the compression resistance of the powder increases, the compression performance deteriorates, and the elastic aftereffect of the billet increases. If lubricants or forming agents are not used to improve its compression performance, the resulting density and strength of the compression will inevitably decrease.
The oxygen content in metal powders exists in the form of compounds or surface adsorption, and sometimes also exists in the form of irreducible impurities. When the powder is not fully reduced or left for too long after reduction, the oxygen content will increase and the compression performance will deteriorate.
(4) The influence of powder particle size and particle size composition. When the particle size and particle size composition of the powder are different, the behavior during the pressing process is inconsistent. Generally speaking, the finer the powder, the poorer its flowability, and the more difficult it is to fill narrow and deep mold cavities, making it easier to form bridges. Due to the fine powder, its loose density is low, and the filling volume in the mold is large, requiring a larger mold cavity size. In this way, the movement distance of the die and the internal friction between the powders will increase during the pressing process, resulting in an increase in pressure loss and affecting the uniform distribution of the compact density. Compared with coarse powders of the same shape, fine powders have poorer compressibility and better formability, due to the increased contact area between the particles of the fine powder. For spherical powders, the particle size of the powder has almost no effect on the density within a medium or large pressure range.
(5) The influence of powder shape. The shape of the powder has the greatest impact on the filling of the mold cavity. Powder with a smooth and regular surface, close to a spherical shape, has good flowability and is easy to fill the mold cavity, resulting in a uniform density distribution of the compact; However, filling powders with complex shapes is difficult and prone to bridging phenomena, resulting in uneven density of the compact due to uneven powder loading. This is particularly important for automatic pressing, as the powders used in production are often irregularly shaped. In order to improve the flowability of the mixture, granulation treatment is often required.
(6) The influence of loose packing density of powder. When the loose density is small, the height of the mold and the length of the punch must be large. When pressing high-density blanks, if the size of the blank is long, the density distribution is prone to unevenness. However, when the loose packing density is low, the contact area of the powder increases during the pressing process, and the strength of the compact is high, which is its advantage.
Second. The influence of lubricants and forming agents on the pressing process
1) The influence of lubricants and forming agents on the pressing process
Different metal powders must use different lubricants or forming agents.
Has appropriate viscosity and good lubricity, and is easy to mix evenly with powder materials.
No chemical reaction occurs with powder materials, making it easy to eliminate and leaving no harmful impurities during pre firing or sintering.
The loose density and flowability of the mixed powder are not significantly affected. Except for special circumstances, its softening point should be high to prevent melting due to temperature rise during the mixing process.
There is no adverse effect on product performance and appearance after sintering.
(2) The dosage and effect of lubricants and forming agents
The amount of lubricant and forming agent added is related to the type and particle size of the powder, the pressing pressure, and the friction surface area, as well as their own materials. The amount of lubricant added is approximately proportional to the shape factor.
Third. The influence of suppression methods on the suppression process
During the compression process, two parameters need to be selected, one is the pressurization speed and the other is the holding time. (1) The impact of pressurization methods. Due to pressure loss during the pressing process, uneven density of the billet may occur. To reduce this phenomenon, bidirectional pressing, multi-directional pressing, or changing the die structure can be used. For parts with complex shapes, composite die punching can be used during forming.
(2) The impact of pressure holding time. During the pressing process, if the powder is maintained at a specific pressure for a certain period of time, it can often achieve very good results.
SAT NANO is a best supplier of metal powder in China, we can offer silver powder, copper powder, gold powder and alloy powder, if you have any enquiry, please feel free to contact us at admin@satnano.com
Polyvinyl alcohol (PVA) is one of the most important and widely used water-soluble polymers in industrial applications. Its preparation process involves first polymerizing vinyl acetate (VAc) to form polyvinyl acetate (PVAc). The vinyl acetate groups (-OAc) on the PVAc are then converted to hydroxyl groups (-OH) through an alcoholysis (hydrolysis) reaction. Based on the degree of alcoholysis, PVA is divided into two major series: fully hydrolyzed and partially hydrolyzed.

Fully hydrolyzed 99 series PVA (such as ElephChem pva 2699, 2499, 2099, and 1799) refers to grades with a degree of hydrolysis of 99.0 mol% or higher. This extremely high degree of hydrolysis is the core prerequisite for these PVA grades to achieve high performance, strength, and water resistance. This blog will analyze, from four perspectives: molecular structure, grade differentiation, performance advantages, and key application areas, how fully hydrolyzed 99 Series PVA has become the cornerstone of "hardcore" materials such as high-performance fibers, specialty films, and durable adhesives.
1.Molecular Structure Determine Performance: The Mechanism and Effect of Complete Hydrolysis
1.1 Hydroxyl Density and Hydrogen Bonding Network Construction
In the fully hydrolyzed 99 Series, nearly all hydrophobic vinyl acetate groups on the molecular chain are replaced by hydrophilic hydroxyl groups. Hydroxyl groups (-OH) are extremely polar functional groups that form strong intramolecular and intermolecular hydrogen bonds, building a highly dense and stable three-dimensional network.
This dense hydrogen-bonding network contributes to two crucial molecular effects:
- High crystallinity: Strong hydrogen bonding forces enable PVA molecular chains to stack neatly and tightly, forming highly ordered crystalline regions. This increased crystallinity is the fundamental reason for the high tensile strength and high modulus of 99 Series PVA.
- Water Resistance: The dense hydrogen bond network makes it difficult for external water molecules to penetrate the crystals at room temperature and disrupt the connections between the molecular chains, effectively preventing PVA from dissolving. Therefore, 99 series PVA is essentially insoluble in water at room temperature and typically requires hot water above 90°C to fully dissolve and disperse. This ensures its structural stability in humid environments and aqueous systems.
1.2 Linear Correlation between Degree of Polymerization and Viscosity/Strength
Assuming a constant degree of hydrolysis (HD>99.0%), the differences between fully hydrolyzed 99 series PVA grades are primarily determined by the average degree of polymerization (DP) or average molecular weight (MW). DP is a key parameter that determines the rheological properties of polymer solutions and the mechanical properties of the final product.
The DP ladder of ElephChem 99 series grades (based on the average DP):
- Ultra-High DP (Polyvinyl Alcohol 2699): DP = 2600-3000. These grades have the longest molecular chains and the highest degree of chain entanglement. Its highest solution viscosity imparts exceptional cohesive strength and adhesion to the cured material, making it an ideal choice for manufacturing high-strength, high-modulus fibers and specialty high-viscosity adhesives.
- Medium-high degree of polymerization (Polyvinyl Alcohol 2499 / Polyvinyl Alcohol 2099): DP = 2,000-2,500. This grade offers a balanced viscosity and mechanical properties. It is the most widely used grade for sizing agents in the textile industry and for general-purpose, high-performance coatings and films.
- Medium-low degree of polymerization (Polyvinyl Alcohol 1799): DP = 1,700-1,800. Its relatively low solution viscosity facilitates its use in systems with high solids content or requiring rapid penetration. For example, precursors for polyvinyl butyral (PVB) require precise molecular weight control (e.g., 1799 for PVB, MW = 76,000-82,000) to ensure efficient acetalization and the quality of the resulting interlayer film.
2. Core Performance Advantages of the Fully Hydrolyzed PVA 99 Series
- Excellent Mechanical Properties (High Strength, High Modulus): High crystallinity gives PVA high tensile strength and modulus. Wet or dry-wet spinning yields high-strength, high-modulus PVA fibers with properties comparable to those of ultra-high-density polyethylene (UHMWPE). These fibers are a key raw material for replacing asbestos in cement reinforcement and ballistic materials.
- Excellent Gas Barrier Properties: PVA films, particularly those produced from the 99 series, offer one of the best barrier properties against gases like oxygen and nitrogen among known polymer materials. The highly hydrogen-bonded network within their molecular structure prevents gas permeation, making them ideal as high-performance barrier layers for oxygen-sensitive food and pharmaceutical packaging.
- Chemical and Oil Resistance: The 99 series PVA shows good resistance to solvents, oils, greases, and weak acids and bases because its molecules are very stable and it has few non-crystalline areas. This makes it useful for industrial coatings and special glues.
- Thermal stability: High crystallinity gives 99 series PVA a higher glass transition temperature (Tg) and melting temperature (Tm), improving the material's resistance to heat deformation and upper temperature limit.
3. Analysis of Key Industrial Applications of Fully Hydrolyzed 99 Series PVA
The unique properties of 99 Series PVA make it irreplaceable in multiple high-value-added sectors:
3.1 High-Strength High-Modulus PVA Fiber (HTHM PVA Fiber)
This is one of the most valuable end-products of 99 Series PVA. For example, the 1799 grade, with a DP of approximately 1750, achieves a high degree of molecular orientation through specialized spinning, heat treatment, and stretching processes.
- Applications: Used to replace asbestos and steel mesh in construction, it reinforces cement, mortar, and concrete, significantly improving the material's impact resistance, freeze-thaw resistance, and fatigue resistance. It is widely used in civil engineering structures such as highways, water conservancy projects, tunnel linings, and cement slabs.
3.2 Textile and Paper Industry
- Textile Warp Sizing: High-polymerization grades such as 2499 and 2699 provide an extremely tough and smooth size film, significantly improving the abrasion resistance and breaking strength of warp yarns during weaving. They are the preferred size for high-density, high-count fabrics (such as denim and premium cotton).
- Papermaking Surface Sizing Agent: As a surface sizing agent, the 99 series PVA forms a high-strength film on the surface of paper, significantly improving its surface strength, folding resistance, and printability. This is crucial for high-end coated paper and specialty functional papers (such as thermal paper and dust-free paper).
3.3 Polyvinyl Butyral (PVB) Precursor
PVB is a core material for automotive safety glass and architectural laminated glass. As an intermediate in the acetalization reaction, the quality of PVA directly determines the optical clarity, toughness, adhesion, and aging resistance of the final PVB film. Grades: 1799 specialty grades (such as SX-I/II/III) with a DP ≈ 1700-1850 are precisely designed to ensure ideal molecular structure and uniform dispersion during the subsequent acetalization reaction, meeting the stringent optical quality requirements of safety glass.
3.4 High-Performance Building Adhesives and Dry-Mix Mortars
In the construction industry, 99-series PVAs are used as high-performance additives to improve material durability and adhesion.
- Applications: As secondary dispersing binders and water-retaining agents in mortars and putty powders, their high bond strength and water resistance ensure the stability and durability of wall putties, tile adhesives, and other materials in humid and temperature-stable environments.
4. Conclusion: Future Outlook for Fully Hydrolyzed 99-Series PVA
99-series PVAs are a classic and promising branch of polymer materials science. By precisely controlling the degree of hydrolysis and polymerization, as demonstrated by ElephChem's grade system, the industry can develop specialized grades tailored to meet the demands of diverse and demanding applications.
From high-strength fibers that reinforce modern infrastructure, to PVB interlayer films that ensure safety, to environmentally friendly, high-performance coatings that enhance quality of life, the 99 series PVA, with its unparalleled strength, stability, and water resistance, continues to play a key role as a driver of high-performance, "hardcore" materials in the upgrading and sustainable development of the global manufacturing industry. As novel uses, like 3D printing and medical hydrogels, ask for better PVA, studies into improving and changing the 99 series PVA will likely increase. This will probably expand its value in industry and its market potential.
Website: www.elephchem.com
Whatsapp: (+)86 13851435272
E-mail: admin@elephchem.com
The core of polyvinyl alcohol (PVA) performance lies in its degree of hydrolysis. The 88 Series PVA, which is partially hydrolyzed (usually around 87.0 to 89.0 mol%), differs from the fully hydrolyzed 99 Series in that it provides better flexibility, interfacial activity, and water solubility that can be adjusted.
When PVA is partially hydrolyzed, about 11% to 13% of vinyl acetate groups (-OAc) are kept in the molecular chain. Because of these hydrophobic groups, the 88 Series PVA acts as an amphiphilic substance with high interfacial activity, unlike the 99 Series. Because of this, it works well as a protective colloid in emulsion polymerization and as a flexible base for strong adhesives and coatings with specific functions.

1. Molecular Structure Determines Function: Amphiphilicity and Protective Colloid Mechanism
1.1 Amphiphilicity Due to Hydrophobic-Hydrophilic Balance
Partially hydrolyzed 88 series PVA molecular chains possess two functional groups with vastly different polarities:
- Hydrophilic groups: A large number of hydroxyl groups (-OH).
- Hydrophobic groups: A small number of evenly distributed vinyl acetate groups (-OAc).
This structure makes PVA a highly effective high-molecular-weight surfactant or protective colloid. When dissolved in water, the molecular chains adsorb at the water-oil (monomer) interface, with the hydrophobic groups tending to embed into the oil phase, while the hydrophilic groups extend toward the water phase. This unique arrangement forms a stable, high-molecular-weight physical barrier (i.e., a protective steric barrier) around the oil phase particles, effectively preventing aggregation of emulsion particles during polymerization, storage, or mechanical shear, and is the core mechanism for ensuring emulsion stability.
1.2 Reduced Crystallinity and Improved Water Solubility
Unlike the highly regular structure of the 99 series, the irregular distribution of vinyl acetate groups on the molecular chain disrupts the regular packing of PVA molecules, resulting in:
- Reduced crystallinity: The proportion of crystalline regions decreases, weakening the hydrogen bond network.
- Improved cold-water solubility: Lower crystallinity allows water molecules to more easily penetrate and disrupt the amorphous region structure. Therefore, 88 series PVA can dissolve quickly or even completely at lower temperatures (typically 40°C to 60°C), greatly simplifying dissolution operations during formulation and production.
2. Effect of Degree of Polymerization on Rheological Properties and Stability
Given a consistent level of partial hydrolysis, the key differences between different PVA grades are mainly in their average degree of polymerization (DP) or molecular weight (MW). The DP has a direct impact on the viscosity of the PVA solution, the thickness of the steric barrier layer, and how the emulsion ultimately performs.
The refined positioning of ElephChem's 88 series grades:
| ElephChem PVA | Average degree of polymerization | Average molecular weight | Core application positioning |
| 2688 / 2488 | 2400~2650 | 118000~130000 | High molecular weight: Provides the strongest steric protection and is used in emulsion polymerizations requiring the highest stability (such as high-performance VAE emulsions). |
| 2088 / 1788 | 1700~2100 | 84000~104000 | General purpose: Balances viscosity and protection for general-purpose PVAc and VAE emulsions and adhesives. |
| 1792 | 1700~1800 | 54000~60000 | Medium-low molecular weight: Suitable for specialty water-soluble fibers and viscosity-sensitive coating systems. |
| 0588 / 0488 | 420~650 | 21000~32000 | Ultra-low molecular weight: Minimal effect on solution viscosity, suitable for inks, inkjet coatings, or as a co-stabilizer in emulsions. |
- High degree of polymerization (Polyvinyl Alcohol 2688 / Polyvinyl Alcohol 2488): Long molecular chains provide a stronger steric hindrance. In emulsion polymerization, long chains help distribute and stabilize monomer droplets and polymer particles, which is needed for high-solids, high-viscosity emulsions.
- Ultra-low degree of polymerization (Polyvinyl Alcohol 0488 / Polyvinyl Alcohol 0588): These stabilizers function similarly to small-molecule emulsifiers, but provide improved polymer adhesion. Their low viscosity allows them to be used in high-solids coatings and slurry systems without affecting the rheological properties of the final product.
3. Analysis of Key Industrial Applications of Partially Hydrolyzed 88 Series PVA
The interfacial activity and controllable water solubility of the 88 series PVAs give them core competitiveness in the fine chemicals, adhesives, and specialty materials sectors:
3.1 Emulsion Polymerization Industry: Stabilizers and Protective Colloids
This is the core and irreplaceable application of the 88 series PVAs. It is widely used in the polymerization of monomers such as vinyl acetate (VAc), acrylates, and styrene-acrylates, and is a key additive in the manufacture of PVAc, VAE, and acrylate emulsions.
- Mechanism: 88 Series PVA acts as a protective colloid, not only stabilizing the emulsion during the initial polymerization phase but, more importantly, determining the freeze-thaw resistance, mechanical shear stability, and rewettability of the final emulsion.
- Applications: Architectural coating emulsions (such as interior wall latex paint), wood adhesives (white latex), textile nonwoven adhesives, carpet adhesives, etc.
3.2 Water-Solubility and Functional Films/Fibers
The low crystallinity of partially hydrolyzed PVA makes it easier to dissolve quickly in cold water, making it a preferred environmentally friendly packaging material.
- Water-Soluble Packaging Film: Used for quantitative packaging of products such as pesticides, dyes, detergents, and laundry detergent beads. Upon application of water, the film quickly dissolves, releasing the contents, providing both convenience and environmental friendliness.
- Water-Soluble Fiber: Used in the textile industry as temporary support yarn or "sacrificial" yarn. After the fabric is finished, the PVA fibers dissolve in warm water, leaving behind a fabric with a special openwork or structural effect.
3.3 Adhesive and Coating Systems
- Adhesives: Due to the retention of hydrophobic groups in the molecular chain, 88-series PVA has better affinity and adhesion to certain hydrophobic surfaces and organic materials than 99-series PVA. It is widely used in specialty paper adhesives and rewettable adhesives (such as postage stamp adhesives).
- Specialty Coatings: Ultra-low molecular weight grades (such as 0488) can be used as ink-receiving coating additives for inkjet printing paper, providing excellent pigment binding and fast drying properties without significantly increasing coating viscosity.
3.4 Other Fine Chemical Applications
- Suspension Polymerization Dispersant: Used in the suspension polymerization of PVC resins, it helps control the size, porosity, and density of PVC particles, which is crucial to the processing properties of PVC resins.
- Ceramic Binder: Used as a temporary binder for bonding ceramics before molding and sintering. After sintering, it can be completely burned and vaporized, leaving no residue.
4. Conclusion: Continuous Innovation in Partially Hydrolyzed 88 Series PVA
ElephChem partially hydrolyzed 88 Series PVA takes full advantage of both hydrophilic and hydrophobic elements in its molecular structure. This allows for careful control during emulsion polymerization and affects how well it sticks and dissolves in water. If the 99 Series is the "rebar" of structural materials, then the 88 Series is the "stabilizer" and "flexibility controller" of fine chemical systems. Partially hydrolyzed 88 Series PVA is still critical to the growth of modern fine chemicals and sustainable materials. This is due to the continued expansion of markets, like those for green water-based coatings, good emulsions, and biodegradable packaging, along with PVA's special interfacial chemistry and grade system.
Website: www.elephchem.com
Whatsapp: (+)86 13851435272
E-mail: admin@elephchem.com
As electronic equipment continues to evolve towards miniaturization, high integration, and increased power density, the service life of these devices is receiving growing attention. When the heat generated by electronic components during operation cannot be dissipated promptly, it leads to localized heat accumulation within the components, severely impacting the normal functioning of the electronic equipment. Particularly for high-power electrical devices, lifespan degradation primarily stems from interfacial heat dissipation issues in electronic components. Therefore, Thermal Interface Materials (TIMs) possessing both high thermal conductivity and excellent electrical insulation are crucial for ensuring their efficient operation.
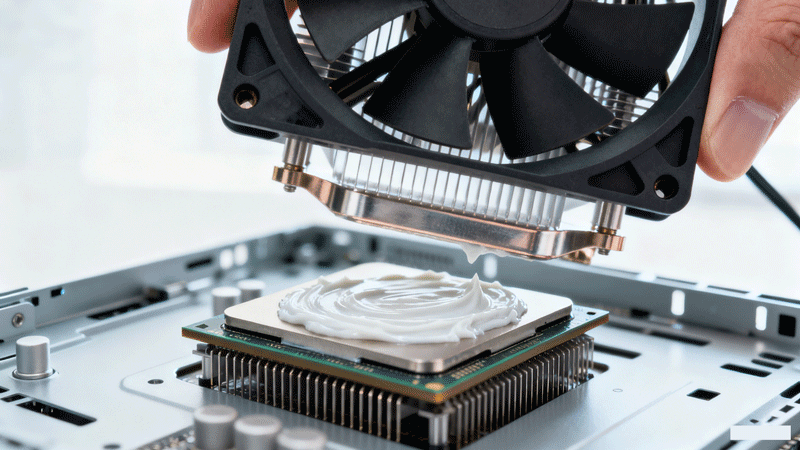
Polymer materials are commonly used as thermal interface materials in electronic components. However, the thermal conductivity of most polymer materials is below 0.5 W·m⁻¹·K⁻¹, which significantly hampers the heat dissipation efficiency of the devices. A prevalent method to enhance the thermal conductivity of polymer materials involves incorporating fillers with higher intrinsic thermal conductivity into the polymer matrix. Ceramic fillers are currently the most widely used thermally conductive fillers in thermally conductive and electrically insulating polymer composites. Commonly used inorganic thermally conductive fillers mainly include:
Oxides: Al₂O₃, ZnO, MgO, SiO₂, BeO
Nitrides: AlN, BN, Si₃N₄
Carbides: SiC
Incorporating these inorganic ceramic fillers into a rubber matrix enables the production of thermally conductive and electrically insulating materials with good overall performance. These materials show broad application prospects in fields such as electronic packaging, thermal management, energy storage, cables, and heat sinks.

About Xiamen Juci Technology Co., Ltd.
Xiamen Juci Technology Co., Ltd. is dedicated to the R&D, production, and sales of high-performance Aluminum Nitride (AlN) ceramic fillers. We offer a comprehensive range of particle sizes, from 1 to 120 microns, to meet diverse application needs. Our AlN fillers are characterized by excellent thermal conductivity and high sphericity, ensuring superior performance in thermal management solutions. It is your ideal partner for high-performance, insulating thermal interface materials.
Contact us:
Xiamen Juci Technology Co., Ltd.
Phone: +86 592 7080230
Email: miki_huang@chinajuci.com
Website: www.jucialnglobal.com
Polyvinyl alcohol (PVA), an indispensable water-soluble polymer material, is used in a wide range of fields, including construction, textiles, papermaking, and chemicals. Among the many PVA specifications, mesh size, or particle fineness, is a key factor in determining processing efficiency and final product quality.
1. Mesh Size Basics: A Measurement of Particle Size
Mesh size is a unit of measurement for powder particle fineness. It refers to the number of holes in a sieve per inch. The smaller the mesh size, the larger (coarser) the particles.
- Mesh size and dissolution rate: The dissolution process of a powder begins with the wetting and penetration of the particle surface by water molecules. The finer the particle size (the larger the mesh size), the greater its specific surface area. A larger specific surface area means that water molecules can contact more PVA molecular chains, significantly accelerating wetting, swelling, and disentanglement, ultimately increasing dissolution rate.
- Mesh size and dispersion uniformity: Fine particles are more easily dispersed in liquid or solid mixtures. When coarse particles (such as 20 mesh) are added to water, they are more likely to settle or clump due to density differences, forming "fish eyes" that are difficult to dissolve.
- Mesh Size and Dust Density: The finer the particle size, the lower the critical velocity at which it becomes suspended in air, resulting in higher dust levels. 20 mesh PVA produces low dust, while 200 mesh PVA requires strict dust control measures.
2. Introduction and Application of PVA Specifications of Different Mesh Sizes
| Mesh Size | 20 mesh(Polyvinyl Alcohol 0588) | 120 mesh (PVA 088-05S) | 200 mesh (POVAL 22-88 S2) |
| Photo |  |
 |
 |
| Bulk Density | Relatively high | Medium | Relatively low (fluffy powder) |
| Key Features | The largest particles have the lowest surface area. This dissolution process is the slowest, but dust generation during operation is minimal; it is also known as a "low-dust" or "dust-free" grade. | This medium-sized particle size is the most commonly used grade in industry. It strikes a good balance between dissolution efficiency, ease of operation, and cost. | The extremely fine particles and maximum surface area ensure the fastest dissolution and the best dispersibility. |
| Applications |
Dry-mix mortar for construction: Coarse-grained PVA, as a binder, is less likely to form high-viscosity clumps during initial mixing, allowing for better dispersion in other components (such as cement and sand). It also produces minimal dust, improving the on-site construction environment.
Specialized slow-release adhesives: In certain specialized construction mortars or adhesives, PVA needs to dissolve slowly to provide lasting adhesion.
Preventing rapid thickening: Suitable for formulations that require prolonged mixing and where rapid thickening of the solution is undesirable. |
Conventional adhesives: Used in the manufacture of common water-based adhesives such as wood glue and paper glue.
Textile sizing agents: Prepare sizings at standard temperatures and times to meet the sizing requirements of most textiles.
Emulsion polymerization protective colloids: Serves as stabilizers and protective colloids in the polymerization of emulsions (such as VAE and acrylic emulsions). They provide a sufficiently rapid dissolution rate without excessively increasing system viscosity, ensuring stability and particle size distribution during emulsion polymerization. |
High-end water-based coatings: Suitable for high-end paints and putty powders that require extremely high dispersibility and a minimum of residual particles.
Fast Preparation/Low-Temperature Dissolution: Fine powder ensures rapid and thorough dissolution of PVA at low temperatures or under limited stirring capacity.
Water-Soluble Film: Used in the production of water-soluble packaging films requiring high transparency and good solubility, such as laundry bags and pesticide packaging.
Pharmaceutical/Cosmetic Excipients: Used in certain fine chemical applications requiring high precision. |
3. How to Make the Best Choice?
Choosing the right mesh size for PVA is essentially a trade-off between production efficiency, environmental safety, and product performance:
For those seeking dissolution speed and product fineness (e.g., coatings and films): 200 mesh is preferred.
For those seeking versatility, balanced performance, and moderate cost (e.g., conventional adhesives): 120 mesh is preferred(PVA 088-50S).
For those emphasizing operational safety, low dust generation (e.g., large-volume batching), or specific sustained-release requirements: 20 mesh is preferred(Poval 217).
Website: www.elephchem.com
Whatsapp: (+)86 13851435272
E-mail: admin@elephchem.com
Polyvinyl alcohol (PVA) is an essential polymer material in numerous applications, including dry-mix mortar, adhesives, and textile sizing. When selecting PVA products, users often focus on their degree of polymerization, alcoholysis degree, and mesh size to ensure core properties such as solubility, viscosity, and bond strength. However, dust content is a crucial, often overlooked indicator that directly impacts production safety, operator health, and material loss. The mesh size of PVA (e.g., 20, 120, 200 mesh) determines its particle size, and particle size is the primary factor determining dust content.
1.Why does PVA generate dust?
The dust content of PVA powder is primarily affected by its particle fineness (mesh size) and morphology:
Finer particles generate higher dust content. Products with larger mesh sizes (e.g., 200 mesh) have a higher proportion of fine particles and a greater ability to remain suspended in air, resulting in greater dust generation. Static electricity: Dry PVA powder is prone to static electricity during friction and conveying, which can exacerbate the suspension and dispersion of fine particles.
2. Definition and Significance of Dust Content
"Dust content" refers to the degree of fine dust suspended in the air during the handling of powder products due to their extremely fine particles. These fine particles (typically less than 10 μm or even 5 μm) not only cause material loss but, more importantly, impact operational safety, environmental cleanliness, and worker health.
Dust analysis of PVA products with different mesh sizes:
| Mesh Size | 20 mesh (PVA 088-05) | 120 mesh (PVA 088-50S) | 200 mesh (PVA-217S) |
| Particle Size Range | Approximately 800-900 μm | Approximately 100-150 μm | Approximately 50-80 μm |
| Particle Surface Area | Very Low Moderate | Moderate | Very High |
| Dust Level (Relative) | Low | Medium-Low | High |
| Photo |
|
 |
 |
| Aerodynamic Characteristics | Heavy particles with high inertia settle easily and are difficult to suspend. | 120 mesh (CCP BP-24S) settle quickly, but will still fly at the moment of feeding. | Light particles are easily carried by air currents and remain suspended for a long time, forming a dust cloud. |
| Occupational Health Risks | Lowest risk. Dust is mostly non-inhalable and has minimal respiratory irritation. | Risk is manageable. General local exhaust ventilation and protective equipment are required. | Highest risk. Fine dust poses a high risk of lung entry and requires strict protection. |
| Dust Explosion Risk | Large particle size makes dust cloud formation difficult, resulting in a low risk. | Possesses some potential for dust cloud formation, resulting in a medium risk. | Dust cloud density easily reaches the lower explosion limit, resulting in the highest risk. |
| Production and feeding requirements | General ventilation is sufficient. | Local exhaust or dust hoods are required. | Efficient, enclosed feeding and specialized dust collection systems are essential. |
| Cost Factors | No additional dust suppression treatment is required. | Anti-caking agents (or granulation) may be required to reduce dust. | High costs must be invested in crushing, fine grading, and safety protection systems. |
|
Properly controlling PVA dust levels is not only a safety requirement but also directly impacts production efficiency and product quality: Excessive dust concentrations can cause material loss and metering errors; Suspended particles entering the reaction system can lead to unstable emulsion polymerization or uneven film thickness; Dust deposition can accelerate equipment wear and affect long-term operational reliability. |
|||
Regardless of mesh size, all PVA powder handling practices should adhere to the following basic principles:
Avoid vigorous handling: Pour the material into the container slowly and steadily, avoiding pouring from a height to minimize interparticle friction and air turbulence. This is the simplest and most effective way to reduce dust generation.
Maintain ventilation in the work area: Local exhaust or exhaust systems must be installed near all feed ports and mixing equipment to capture generated dust at the source.
Adhere to chemical management practices: Although PVA has low toxicity, the storage, handling, and emergency response instructions in the Material Safety Data Sheet (SDS) should still be reviewed and followed.
Environmental cleanliness: Regularly clean accumulated dust from equipment and floors with an industrial vacuum cleaner. Never use compressed air to blow dust, as this will re-inflate accumulated dust, increasing the risk of explosion and inhalation.
3. Conclusion
In the production and use of PVA powder, dust management is the intersection of process control and safety assurance. Different mesh sizes require appropriate feeding methods and protective measures. Especially for fine powders above 120 mesh (CCP BP-20S), engineering approaches to dust control should be prioritized, rather than relying solely on personal protection. Through scientific particle size selection, process design, and environmental control, PVA product performance and production stability can be maximized while ensuring safety.
Website: www.elephchem.com
Whatsapp: (+)86 13851435272
E-mail: admin@elephchem.com
Membrane materials technology plays a key role in environmental protection, energy, biomedicine, and other fields. Polyvinyl alcohol (PVA) has become a key target of membrane material research due to its excellent water solubility, film-forming properties, and biocompatibility. However, due to the high concentration of hydroxyl groups in its molecular chains, PVA easily swells or dissolves in high-humidity environments, affecting its stability in complex applications. To overcome these limitations, research on Modified Polyvinyl Alcohol has intensified in recent years. Through chemical cross-linking, blending, and inorganic filler incorporation, the water resistance, mechanical properties, and chemical stability of Polyvinyl alcohol film (PVA film) have been significantly improved. Modified PVA membranes have found widespread application in water treatment, fuel cells, gas separation, and other fields. The rise of green and environmentally friendly modification technologies has given PVA membranes greater potential for biodegradable and environmentally friendly applications. By optimizing production processes and expanding functional modification strategies, PVA membranes will play a more significant role in the field of high-performance membrane materials.
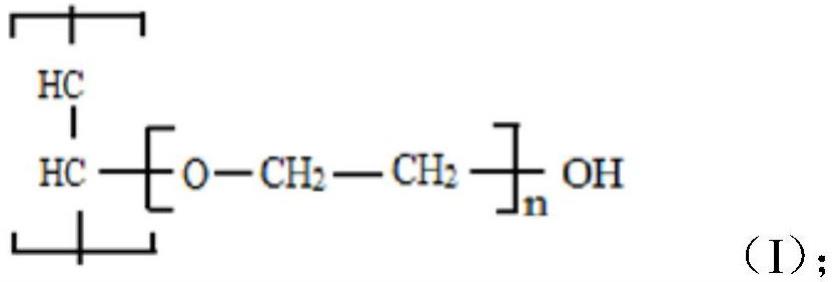
1. Polyvinyl Alcohol Modification Methods
1.1 Chemical Crosslinking
Polyvinyl alcohol (PVA) is a highly polar polymer. Due to the large number of hydroxyl groups on its backbone, it easily forms hydrogen bonds with water molecules, causing it to swell or even dissolve in humid environments. This significantly limits its stability in certain applications. Chemical crosslinking is an effective method. By introducing crosslinks between PVA molecular chains, a stable three-dimensional network is formed, thereby reducing its water solubility and improving its water resistance and thermal stability. Crosslinking typically involves introducing covalent bonds between PVA molecules, making the polymer chains less dispersible in water. Common crosslinking agents include aldehydes (such as glutaraldehyde), epoxides (such as epichlorohydrin), and polyacids (such as citric acid and maleic anhydride). Different crosslinking agents affect the crosslinking pattern and the properties of the modified polymer. For instance, when glutaraldehyde meets PVA's hydroxyl groups in an acidic environment, they create a solid crosslinked structure. Also, maleic anhydride can link PVA sections by esterification, which really helps PVA resist water. Because these cross-linked PVA films have stronger links between molecules, they can handle more heat, as seen by their higher glass transition temperature (Tg) and thermal decomposition temperature (Td).
1.2 Blending Modification
Blending modification is another important method for improving PVA film performance. By blending with other polymers, PVA's mechanical properties, water resistance, and chemical stability can be optimized. Due to PVA's inherently hydrophilic nature, direct blending with hydrophobic polymers may present compatibility issues. Therefore, it is important to select appropriate blending materials and optimize the blending process. For example, when blended with polyvinyl butyral (PVB), PVB's hydrophobicity enables PVA films to maintain good morphological stability even in high humidity environments. Furthermore, PVB's high glass transition temperature improves the heat resistance of the blended films. Blending with polyvinylidene fluoride (PVDF) significantly enhances the hydrophobicity of PVA films. Furthermore, PVDF's excellent chemical resistance allows the blended films to remain stable even in complex chemical environments. PVA can also be blended with polyethersulfone (PES) and polyacrylonitrile (PAN) to enhance the membrane's selective permeability, making it more widely applicable in gas separation and water purification membranes.
2. Application of PVA Modified Membranes in High-Performance Membrane Materials
2.1 Water Treatment Membranes
The development of water treatment membrane technology is crucial for addressing water resource shortages and improving water quality and safety. PVA membranes work really well as films and get along with living tissue, so they could be used in all sorts of membrane separation stuff like ultrafiltration, nanofiltration, and reverse osmosis. But, because PVA loves water and dissolves in it, it can break down over time. This makes the membrane weaker and not last as long. That's why changing up PVA membranes has become a big focus in water treatment research. Chemical crosslinking is a key technology for improving the water resistance of PVA membranes. Crosslinking agents (such as glutaraldehyde and maleic anhydride) form stable chemical bonds between PVA molecular chains, maintaining the membrane's stable morphology in aqueous environments and extending its service life. In addition, the introduction of inorganic fillers is also an important means of improving the hydrolysis resistance and mechanical strength of PVA membranes. Adding nano-silica (SiO₂) and nano-alumina (Al₂O₃) can create a strong mix in the membrane material. This makes the membrane better at resisting breakdown from water and boosts its strength. So, it keeps working well even with high pressure. Also, mixing PVA with other polymers like polyethersulfone (PES) and polyvinylidene fluoride (PVDF) makes the membrane more water-resistant and less prone to fouling. This means it lasts longer and maintains its flow rate, even with dirt buildup.
2.2 Proton Exchange Membranes for Fuel Cells
Fuel cells are clean and efficient energy conversion devices, and proton exchange membranes, as their core component, determine their performance and lifespan. PVA, due to its excellent film-forming properties and processability, is a promising candidate for proton exchange membranes. However, its low proton conductivity in its raw state makes it difficult to meet the high-efficiency requirements of fuel cells, necessitating modification to increase proton conductivity. Sulfonation modification is one of the key methods for improving the proton conductivity of PVA membranes. To boost how well membranes absorb water and help protons move better, we add sulfonic acid to the PVA chain. This makes continuous water channels. Mixing it up can also do the trick. If you mix PVA with SPS and SPEEK, they form a network that helps exchange protons and makes the membrane stronger. But, using PVA membranes in DMFCs has its problems. Methanol can leak through, wasting fuel and making things worse. To fix this, scientists have added things such as sulfonated silica and zirconia nanoparticles to PVA membranes. They also use layers to block methanol from passing through the membrane and reduce leakage.
3. Development Trends and Challenges
3.1 Development of Green and Environmentally Friendly Modification Technologies
With increasingly stringent environmental regulations and the growing adoption of sustainable development concepts, green and environmentally friendly modification technologies for PVA films have become a key research focus. Research on biodegradable PVA films has made significant progress in recent years. By blending with natural polymers (such as chitosan, starch, and cellulose) or introducing biodegradable nanofillers (such as hydroxyapatite and bio-based nanocellulose), the biodegradability of PVA films can be significantly improved, making them more easily decomposed in the natural environment and reducing pollution to the ecosystem. Furthermore, to reduce the environmental and human impact of toxic chemicals used in traditional cross-linking modification processes, researchers have begun developing non-toxic cross-linking agents and more environmentally friendly modification processes. These include chemical cross-linking using natural cross-linkers such as citric acid and chitosan, and physical modification methods such as ultraviolet light and plasma treatment, achieving pollution-free cross-linking. These green modification technologies not only enhance the environmental friendliness of PVA films but also enhance their application value in food packaging, biomedicine, and other fields, making them a key direction for the future development of polymer membrane materials.
3.2 Challenges and Solutions for Industrial Application
Although modified PVA films hold broad application prospects in the field of high-performance membrane materials, they still face numerous challenges in their industrialization. High production costs are a major bottleneck, particularly for PVA films involving nanofillers or special modifications. Expensive raw materials and complex preparation processes limit large-scale production. Process optimization still requires improvement. Currently, some modification methods suffer from high energy consumption and long production cycles, hindering the economic viability and feasibility of industrial production. To address these issues, future efforts will focus on developing low-cost, efficient preparation processes, such as adopting environmentally friendly aqueous synthesis techniques to improve production efficiency, while optimizing the blending system to enhance the performance stability of PVA films. Furthermore, future development directions for high-performance PVA films will focus on improving durability, reducing production energy consumption, and expanding intelligent functionality. For example, developing intelligent PVA films that can respond to external stimuli (such as temperature and pH changes) to meet a wider range of industrial and biomedical needs.
4. Conclusion
Polyvinyl alcohol (PVA), as a high-performance polymer, holds broad application prospects in the membrane material field. PVA films can be made stronger and more resistant to the elements by using methods like chemical cross-linking, co-modification, and adding inorganic fillers. This makes them suitable for things like water treatment and fuel cells. Also, new green modification tech has made PVA films break down easier and be less toxic. This means they could be big in environmental protection and medical uses. In the future, industrial applications will still face challenges in production costs and process optimization. Further improvements in the economic efficiency and feasibility of modification technologies are needed to promote the widespread application of PVA films in the field of high-performance membrane materials and provide higher-quality membrane material solutions for sustainable development.
Website: www.elephchem.com
Whatsapp: (+)86 13851435272
E-mail: admin@elephchem.com
Film-forming agents are important adjuvants in pesticide seed coatings and are key functional ingredients in seed coatings. The inclusion of film-forming agents allows seed coatings to form a film on the seed surface, distinguishing them from other formulations such as dry powders, dispersible powders, liquids, and emulsions. The primary function of the film-forming agent in seed coatings is to adhere the active ingredient to the seed surface and form a uniform, smooth film. Film-forming agents need to be water-resistant to hold up in wet conditions like rice paddies, but they also need to let some water through so seeds can grow. It’s also good if they can soak up a bit of water from the soil, which helps seeds grow when it’s dry. Most polymers are good at one of these things, but not all. For example, it's hard to find something that’s both waterproof and lets water pass through. Right now, seed coatings often use just one polymer, so it’s tough to get all these properties at once. This is a main problem for making better seed coatings for rice fields.
Polyvinyl Alcohol (PVA), with its excellent film-forming, swelling, and water permeability, is currently the most widely used film-forming agent in seed coatings. However, its poor water resistance makes it susceptible to water erosion after seed coating, making it unsuitable for use alone in paddy fields or in high-humidity areas. VAE Emulsion (Vinyl Acetate–ethylene Copolymer Emulsion) exhibits strong water resistance, but VAE films only swell in water, not dissolve, and are impermeable to water. Clearly, VAE alone is also unsuitable as a seed coating agent. To address these issues, we used a solution blending method to prepare a series of blended films using PVA and VAE in varying ratios, hoping to improve the water resistance of Polyvinyl alcohol film (PVA film).
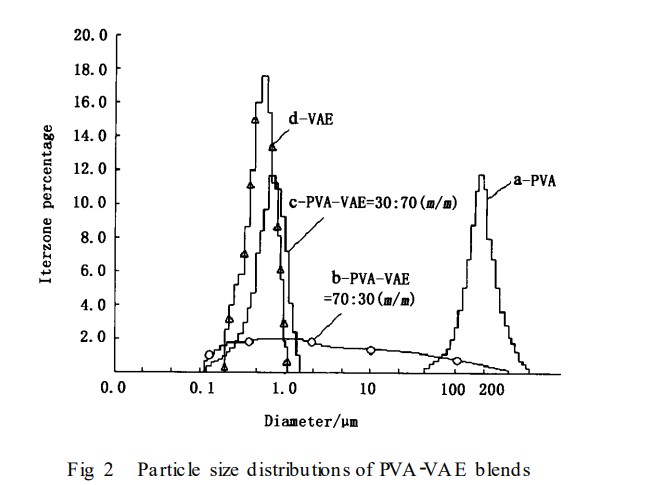
1. Microscopic Observation of the Blend System
Figure 3-a shows that the PVA colloidal particles exhibit distinct micellar behavior, while the VAE colloidal particles exhibit relatively regular spherical shapes with particle sizes ranging from 700 to 900 nm and unclear outlines (Figure 3-b), consistent with literature reports. After blending, the outlines of the PVA and VAE colloidal particles clearly exhibit a core-shell structure (Figure 3-c), indicating that hydrogen bonding within the blend system alters the electron density around the particles. Furthermore, the particles of each phase are evenly distributed within the blend system, with no apparent interface formation, indicating good compatibility.
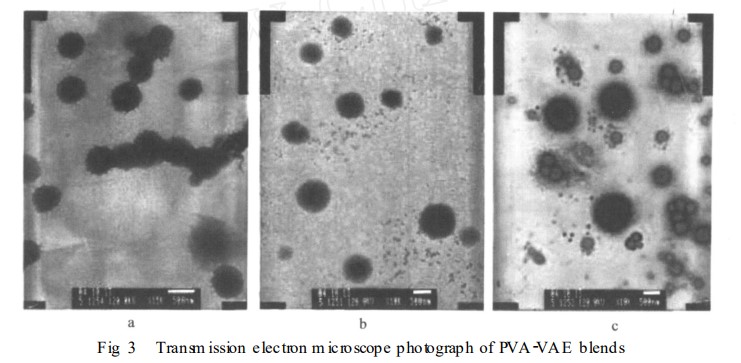
2. Water Resistance and Permeability of the Blend System
The test results for the water permeability of the blend system are listed in Table 1. After the addition of PVA, the water permeability of VAE was significantly improved. The water permeabilities of vp10, vp20, vp30, and vp40 were ideal, meeting the requirements of seed germination and generally consistent with the results of the seed germination test. When we looked at how long it took for water to pass through, we found that as the VAE content went up, it took longer for water to start permeating: 0.2 hours (vp0), 0.25 hours (vp10), 0.5 hours (vp20), 0.75 hours (vp30), 1.2 hours (vp40), 2.5 hours (vp50), and over 6 hours (vp60-100). Except for vp0, all groups lasted the whole 24 hours without dissolving, which shows that adding VAE really made the material more water-resistant. The national standards GB 11175-89 and GB 15330-94 test water resistance and permeability by checking how much the film swells. These tests cannot fully capture the water permeation, water erosion, and subsequent dissolution of seed coating films used in this test. Visual assessment of these indicators is also difficult to accurately determine. The "L-shaped glass tube method" proposed in this paper measures the water permeability and water resistance of latex films. In principle, this method directly measures water permeation, water dissolution, and water solubility. Precise measuring instruments such as automatic samplers and pipettes are used for indicator control. Visual assessment of the "water permeation and dissolution" indicators and time measurements are easily determined. The experimental procedure is simple and can accurately reflect the actual performance of the membrane.
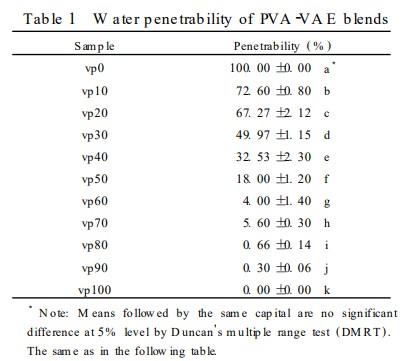
3. Effect of Modified Films on Seed Germination
Rice seed germination tests (see Table 2) showed that blend films with less than 30% VAE didn't really change how well the seeds sprouted, so they should work fine for coating seeds. But, if the VAE is over 70%, the seeds didn't sprout well at all. None of the other samples sprouted well enough after 7 days to meet the standard.
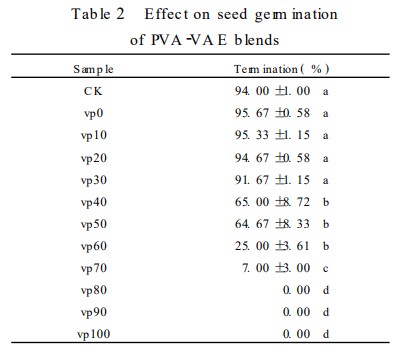
Structural characterization of the blend films revealed good intermolecular compatibility between PVA and VAE after solution blending. The micelles in the PVA solution were opened, and no interface between the two phases was observed, demonstrating the feasibility of using VAE to modify PVA. The performance of PVA/VAE blend films at mass ratios of 80:20 and 70:30 was suitable for rice seed coating applications. Compared with PVA films alone, the introduction of VAE significantly improved the water resistance of the blend films, maintaining suitable water permeability and having no significant effect on seed germination. The method of modifying PVA blends with VAE emulsion is feasible for application in the film-forming agent field of pesticide seed coating agents.
Website: www.elephchem.com
Whatsapp: (+)86 13851435272
E-mail: admin@elephchem.com
- Bismaleimide Series2
- Cross-Linking agent / Vulcanizing Agent1
- Curing Agent1
- Engineering Plastic Pellets4
- Epoxy Resin2
- Ethylene-VinylAlcohol Copolymer(EVOH)1
- Fish Oil1
- Food Additives3
- Glucosamine1
- Heat-resistant modifier series1
- High Assay Quaternary Ammonium Compounds9
- Low Assay Quaternary Ammonium Compounds13
- Modified Polyvinyl Alcohol1
- Monomalemide Series2
- Other Surfactants/Catalysts8
- Plastic Random Packing1
- Plastic Structured Packing1
- Polyacrylamide1
- Polyurethane Resin2
- Polyvinyl Alcohol (PVA)2
- Power Coatings3
- Quaternary Ammonium Hydroxide4
- Special Quaternary Ammonium Compounds7
- TPU4
- Tertiary Amines1
- UV Ink1
- VAE Emulsion (Vinyl Acetate–ethylene Copolymer Emulsion)1
- aluminum paste1
- antiform2
- fire sleeve2
- resin2
